Enabling Shape Memory Biomedical Materials with Micro-Compounding
Published: January 8, 2026 · Reading time: 5 minutes
Introduction
Biomedical materials are rapidly evolving from passive structural components into active, functional systems that can respond to their environment. In modern healthcare, materials are expected to adapt, deploy, degrade, and interact safely with biological tissues. This shift has driven strong interest in smart polymers, materials capable of changing their properties in response to external stimuli such as temperature, pH, light, or mechanical load.
Smart Polymers for Smarter Biomedical Solutions
Among smart polymers, shape memory polymers (SMPs) stand out due to their unique ability to be programmed into a temporary shape and later recover their original geometry when exposed to a specific trigger. Thermally activated SMPs are particularly attractive for biomedical applications because their activation temperature can be engineered close to physiological conditions. This enables minimally invasive insertion of a compact device, followed by controlled deployment once the material reaches body temperature.
For researchers and medical device developers, the challenge is no longer proving that shape memory works, it is about engineering materials that combine reliable actuation with mechanical integrity, biocompatibility, and controlled biodegradation.
Why Shape Memory Matters in Biomedical Devices
Shape memory behavior unlocks entirely new design paradigms for biomedical devices. A device can be inserted in a compact form, reducing surgical trauma, and then autonomously recover its functional shape inside the body. This concept is highly relevant for applications such as self-expanding stents, tissue scaffolds, orthopedic fixation elements, sutures, and minimally invasive implants (Fig. 1).
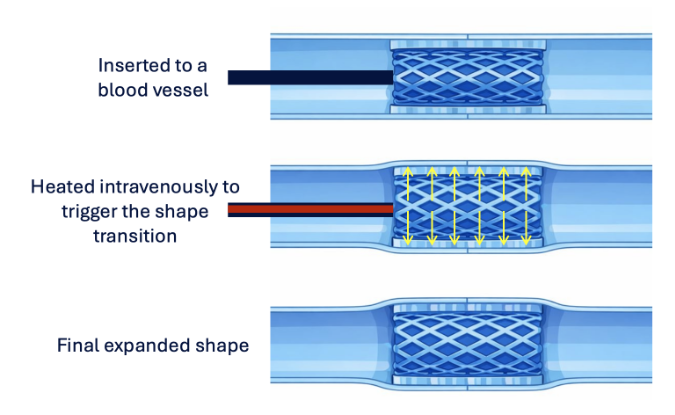
Figure 1. Typical application regarding shape memory stents
However, biomedical shape memory materials must satisfy multiple requirements simultaneously. They must generate sufficient recovery force, activate within a narrow temperature window, maintain performance over repeated cycles, and degrade safely without releasing toxic byproducts. Achieving this balance requires careful control over polymer chemistry, morphology, and processing history [1,2].
This is why formulation development and processing strategy are inseparable when designing shape memory biomedical materials.
PLA-Based Blends: Sustainable and Functional
Poly(lactic acid) (PLA) is one of the most widely used polymers in biomedical applications. Derived from renewable resources, PLA is biodegradable, biocompatible, and already accepted in clinical use. Despite these advantages, neat PLA is inherently brittle and lacks the elasticity required for demanding shape memory applications.
Blending PLA with thermoplastic polyurethane (TPU) offers an elegant and scalable solution. In PLA/TPU blends, PLA typically acts as the switching phase responsible for shape fixation and recovery, while TPU provides elasticity, toughness, and improved deformability. By adjusting the PLA/TPU ratio, it becomes possible to tune mechanical behavior, shape recovery performance, and degradation rate.
This blend-based approach enables the development of sustainable, bio-based shape memory polymers that are both functional and application-ready.
Compatibilization: Enabling Performance and Reliability
A key challenge in PLA/TPU blends is their intrinsic immiscibility, which can lead to weak interfacial adhesion and suboptimal performance. Reactive compatibilization provides a powerful strategy to overcome this limitation. By introducing small amounts of diisocyanate-based chain extenders during melt processing, chemical interactions are promoted between PLA and TPU chains.
This compatibilization dramatically improves interfacial bonding, leading to higher shape recovery ratios, faster recovery kinetics, and improved stability under cyclic actuation. Importantly, compatibilization also slows enzymatic degradation by preventing premature phase separation and structural disintegration (Fig. 2).
From a biomedical perspective, it is critical that these performance gains do not compromise safety. In-vitro studies confirm that compatibilized PLA/TPU systems remain biocompatible and non-cytotoxic, even in the presence of reactive additives [3,4].
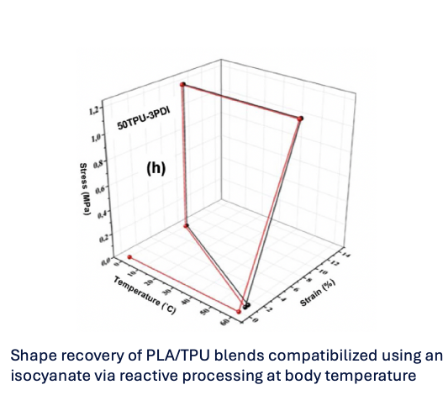
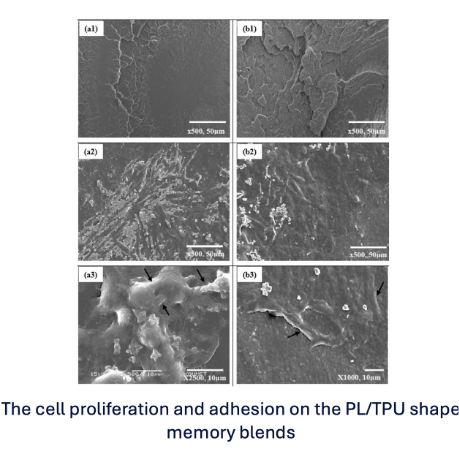
Figure 2. Shape memory behavior with perfect biocompatibility
Processing Perspective: Why Melt Processing Still Matters
While chemistry defines what a material can do, processing defines what it actually does. Melt processing plays a decisive role in determining phase morphology, molecular architecture, and thermal history, all of which directly influence shape memory behavior and degradation kinetics.
Twin-screw compounding followed by injection molding ensures that compatibilization reactions occur under realistic shear and temperature conditions. This makes laboratory-scale results directly relevant to industrial manufacturing, reducing the risk of scale-up failures.
For biomedical materials, where regulatory approval and reproducibility are critical, melt processing provides a robust and scalable pathway from formulation development to final device manufacturing.
Why This Matters for Micro-Processing
Micro-processing technologies fundamentally change how shape memory biomedical materials are developed. Xplore Microcompounders and Micro-Injection Molding systems allow researchers to work with gram-scale material quantities while maintaining full control over processing parameters such as temperature, shear rate, and residence time.
This enables rapid screening of formulations, efficient use of expensive or limited biomedical-grade polymers, and realistic evaluation of reactive compatibilization strategies. Researchers can produce standardized test specimens for mechanical testing, shape memory evaluation, degradation studies, and biocompatibility assessment, all from the same micro-scale processing workflow.
By bridging the gap between academic research and industrial relevance, Xplore micro-processing accelerates innovation, reduces development cost, and de-risks the transition from concept to medical device. For anyone developing next-generation shape memory biomedical materials, micro-processing is not just a convenience, it is a strategic advantage.
References
- Gall, K.; Yakacki, C. M.; Liu, Y.; Shandas, R.; Willett, N.; Anseth, K. S. Thermomechanics of the Shape Memory Effect in Polymers for Biomedical Applications. J. Biomed. Mater. Res., Part A 2005, 73A, 339–348. (LINK)
- Ajili, S. H.; Ebrahimi, N. G.; Soleimani, M. Polyurethane/Polycaprolactone Blend with Shape Memory Effect as a Proposed Material for Cardiovascular Implants. Acta Biomater. 2009, 5, 1519–1530. (LINK)
- Dogan, S. K.; Boyacıoğlu, S.; Kodal, M.; Gökçe, O.; Özkoç, G. Thermally Induced Shape Memory Behavior, Enzymatic Degradation and Biocompatibility of PLA/TPU Blends: Effects of Compatibilization. J. Mech. Behav. Biomed. Mater. 2017, 71, 349–361. (LINK)
- Lai, S. M.; Lan, Y. C. Shape Memory Properties of Melt-Blended Polylactic Acid (PLA)/Thermoplastic Polyurethane (TPU) Bio-Based Blends. J. Polym. Res. 2013, 20, 1–8. (LINK)